Mobile Visit Solutions
Mobile Visit Solutions
Improving patient access, diversity, and retention.
Hawthorne Effect’s proven solution brings new medical devices, pharmaceuticals, and therapies to market with better speed and outcomes

Hawthorne Effect solves for the biggest trial challenges
Distance and time can combine to create patient access limitations and exacerbate attrition, which result in non-representative subject populations and missing data - ultimately jeopardizing regulatory approvals and market adoption.
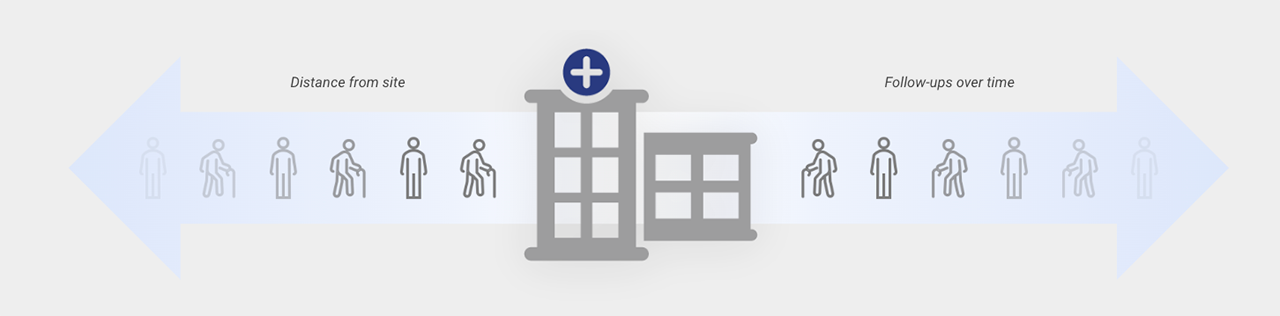

Complexity Unlocked
The Hawthorne Effect Trial Solution
Complex, Distributed Visit Orchestration
We ensure comprehensive investigator oversight by leveraging proprietary, transformational technology to automate complex orchestration and manage distributed touchpoints. The Visit Delivery Platform provides the building blocks to deliver real-time, validated, and highly accurate data for any clinical trial.

In-Person Clinical Touchpoints
We then match complex trial needs to trained and qualified medical professionals, whether leveraging the growing Hawthorne Hero network of 4K+ clinicians or augmenting with your network, to focus on the patient journey.



Cutting-Edge Mobile Assessments
Hawthorne collects the highest quality data by deploying cutting-edge portable technology to perform assessments anywhere and enabling reliable data validation and interpretation.

Increased Patient Access
Expanded geographic catchment areas and patient enrollment from anywhere – even for those with limited mobility
Enabled Investigators
Standardized operations and consistent data quality and capture, extending research to new community providers
Improved Data Quality
Cleaner, faster, representative data that accelerates regulatory submissions and market introduction
Our PROOF
Case Studies
IMPROVED DATA QUALITY & RELIABILITY
Rare disease pharmaceutical study
Hawthorne Effect was brought in as a rescue partner and enabled completion of a Phase III rare disease (recurrent pericarditis) drug trial, leading to FDA approval during a worldwide pandemic.
Study Insights
- COVID-19 shelter-in-place prohibited patient follow-up
- Hawthorne completed the primary endpoint visit for 40% of patients
- Published results in the NEJM during the pandemic
- 96% reduction in the risk of recurrent pericarditis episodes
- Received orphan drug designation for the treatment of recurrent pericarditis

Increased Patient Access
Valvular heart disease prevalence study (PREVUE-VALVE)
In this cardiovascular prevalence study with the Cardiovascular Research Foundation, Hawthorne Effect provided access to representative target populations and accelerated enrollment.
Study Insights
- Collaboration with retail health and academia
- HE single site and end-to-end patient journey
- Eliminated need for 100 sites with one virtual site
- 500 subject pilot enrolled in four months
- 5,000 patient trial underway
- 15% participant age of 80+
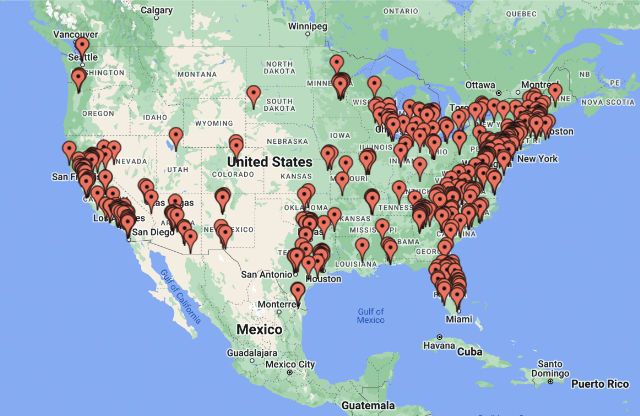
Enabled Investigators
Medical device pivotal trial for tricuspid regurgitation
Hawthorne Effect was a key player in this study for symptomatic patients with severe tricuspid regurgitation who were at greater estimated risk for mortality with tricuspid valve surgery. We helped to accelerate enrollment and enable key sites to exceed recruitment targets.
Study Insights
- Enrollment one month early + expanded catchments
- Last patient followed early
- Accelerated time to reporting and submission
Discover how we can help your trial
Let’s discuss how Hawthorne Effect can take your clinical trial to completion and approval with access and accuracy
