Meet us at WODC 2022 in Boston from July 11-13
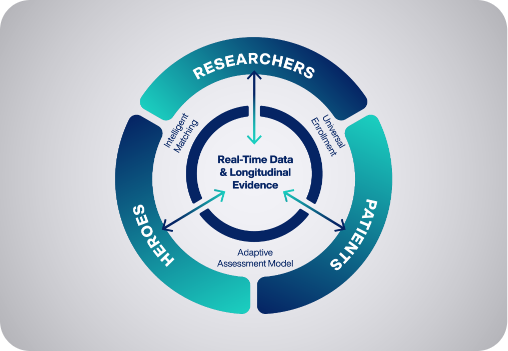
The Hawthorne Effect ACT™ solution accelerates clinical trials by expanding access to new patient populations and optimizing participation.
Hawthorne Effect goes beyond traditional decentralized clinical trials (DCT) models and takes the trial directly to the patient in their home.
HIPAA-compliant and cloud-based SaaS solution
ACT™ provides four key components to manage the clinical trial journey among researchers, clinicians, and trial participants.
Collaboration and flexibility for KOLs and sponsors
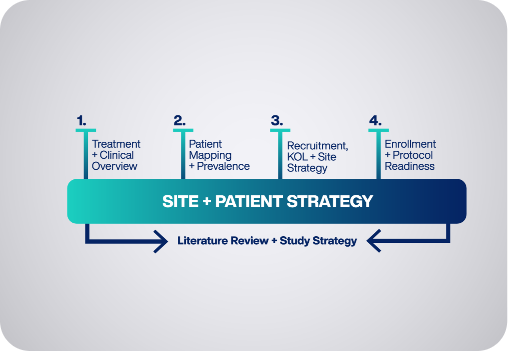
Our four-phase plan combines the ACT™ platform with our network of Heroes and a robust playbook of adaptive processes.
2,500+ Heroes, 50 states, 20K+ visits, millions of assessments
The Hawthorne Network of Heroes represents a broad spectrum of therapeutic areas work and live where the trial is taking place.

Hawthorne Effect brought the RHAPSODY Trial to completion and FDA approval during a pandemic.
- Phase III rare disease drug trial
- Sponsor: Kiniksa Pharmaceuticals
- Sample size: 86 patients (every patient mattered)
- COVID-19 shelter in place prohibited patient follow-up
- Hawthorne completed 30+ in home, complex study visits for 12 academic sites in 15 days
- Drug was approved to market with published results during the pandemic and is now saving lives